
Order No. CSP002
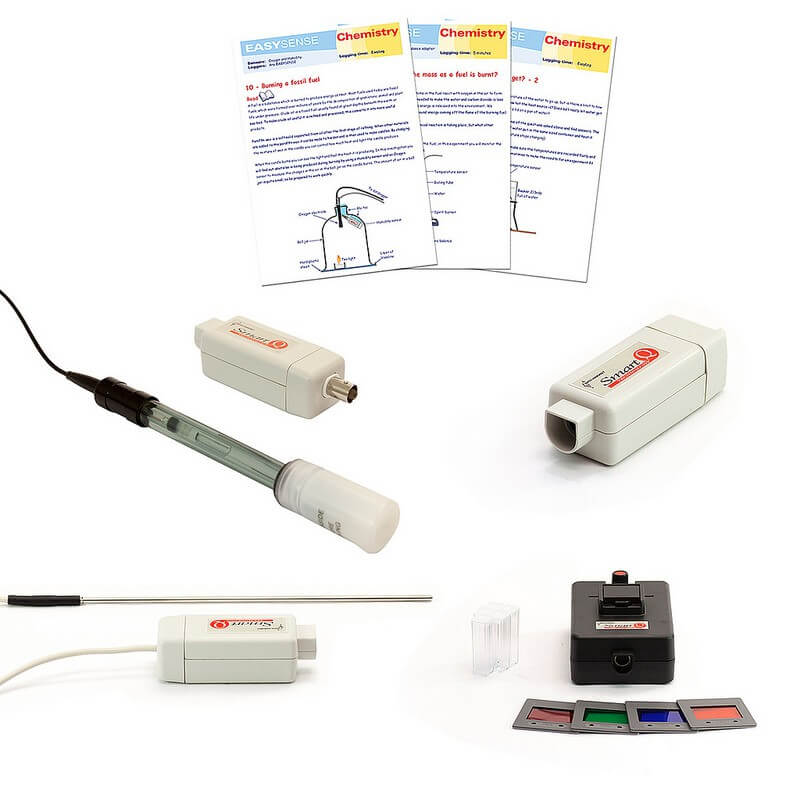
A curriculum pack that includes sensors and eBook curriculum materials. Each pack has been carefully designed to offer maximum flexibility and subject coverage at an affordable price.
All our sensors are supplied with 5 years warranty and lifetime support.
With the included sensors the following experiments can be achieved, note that a suitable Data Logger is not included in the sensor pack.
Chemistry L2 eBook
Chemistry L3 eBook

Learn more about the Chemistry (11-18) Sensor Pack specification and range information.
Specification
| All Sensors | Please see individual sensor pages for specifications |
|---|---|
| All eBooks | Supplied in digital PDF format |
What's in the box
| 1x Gas Pressure - Differential 200kPa |
| 1x pH Pack |
| 2x Temperature Sensors |
| 1x Colorimeter Sensor |
| 1x Chemistry L2 eBook |
| 1x Chemistry L3 eBook |
All our sensors come with 5 years warranty and lifetime support, we ensure your investment gives you complete peace of mind.